Blog
Top 7 End point detection method in Wet granulation

Overview
Wet granulation is undeniably the most preferred method for preparing granules, over the other methods such as dry granulation or Moisture-Assisted Dry Granulation (MADG). Despite being an age-old technique, formulation scientists still struggle with establishment and reproducibility of the correct endpoint during the wet granulation process.
Key indicators in determining endpoint of granulation
Granulation endpoint can be defined as the time when the granules reach the desired properties such as granule size distribution, flowability or bulk density, as stipulated by the formulator. The similar granule properties would consequently give similar tablet properties regardless of the variations in the process parameters; commonly called as the “principle of equifinality”. The goal of end-point detection in a granulation process is to obtain an indication of the formation of granules with the desired properties such as rheology, particle size or porosity. Researchers and pharmaceutical equipment manufacturers have devised several methodologies to provide more reliable results for the end point of the granulation process. However, each of these methods has its own downside, leading to intermittent manual checks of the product by the operators. The latter practice is highly subjective and may involve several inaccuracies. Therefore, it is desirable that an accurate and reproducible end-point detection method is designed which considers the binder addition time as well as the wet mixing time in a wet granulation process (1).
Indicators to determine end point of granulation can be broadly classified in the following categories:
- Manual measurement parameters (granule strength, density)
- Machine readings (current, torque, powder)
- Empirical measurement (mixing time, impeller speed)
End point Detection in Granulation - Methods
Based on the above principles, there are several end-point detection methods which are technically feasible to be used in the pharmaceutical industry (2). All possible methods for end-point detection in a high shear mixer granulator have been described hereafter.
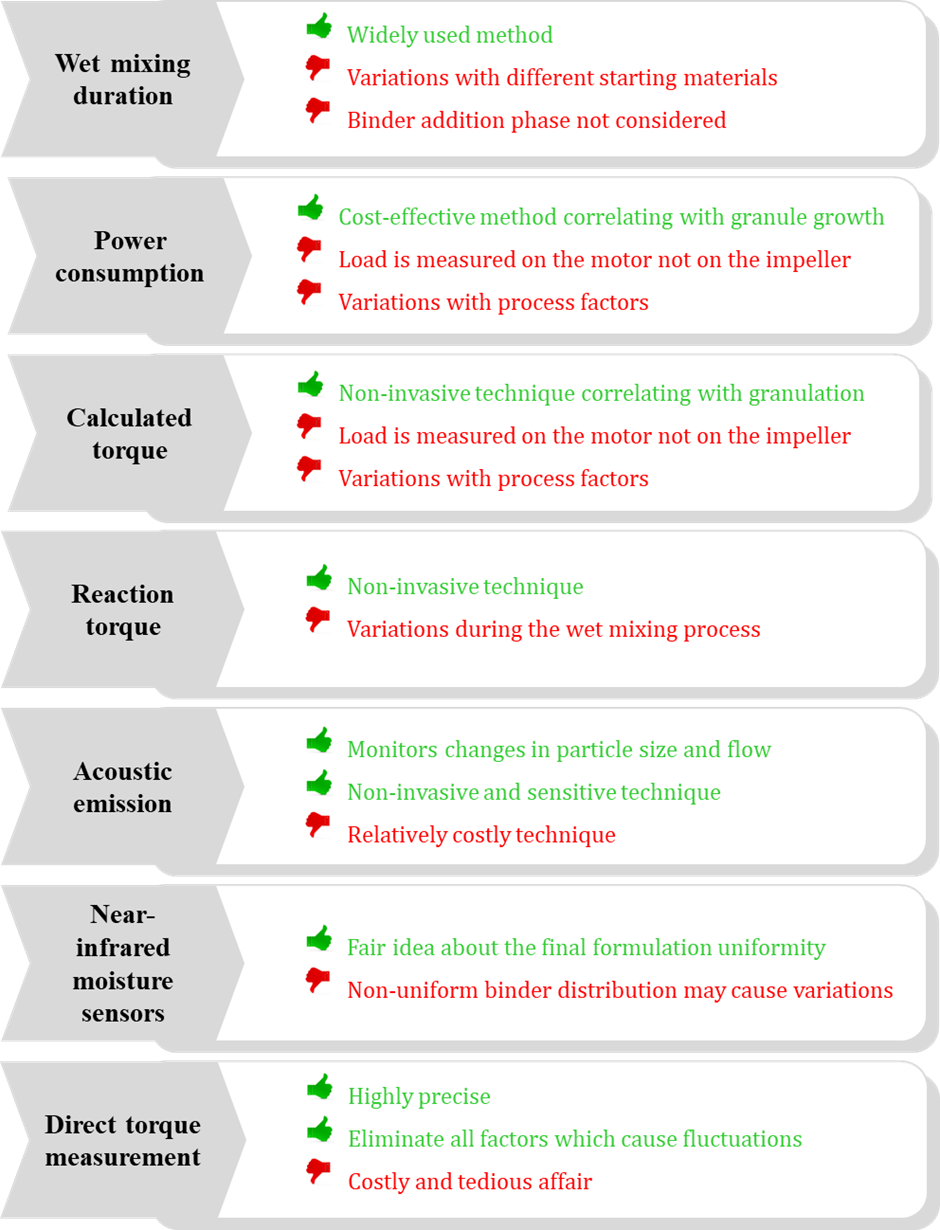
- Wet mixing duration: The duration of wet mixing step in the granulation process is one of the most common and oldest methods for end-point detection in wet granulation. The binder addition phase is followed by the wet mixing phase in the high shear mixer granulator.
- Power consumption: The technique is based on measurement of power consumed by the motor mixer (in amperes). The measured value corresponds to the granule growth and changes with the changes in the consistency of the powder mixer. The increased consistency of the powder mix increases the resistance on the impeller blades which in turn increases the load on the motor.
- Calculated torque: The basic means of evaluation of endpoint in this method is current being supplied during the granulation process which is then calculated to give a torque value.
- Reaction torque: Reaction torque is generated due to the tension in the stationery motor when it tries to move in the opposite direction while the impeller shaft rotates. The tension is generated since the motor is fixed in place and cannot rotate.
- Acoustic emission: This method involves piezo-electric emission sensors which detect the changes occurring during the granulation process such as particle size, flow, compression properties and powder rheology (3).
- Near-Infrared (NIR) sensors: The NIR sensors are used to indicate the moisture content during the granulation process and defines the distribution of wet binder.
- Direct torque measurement: In this method, the torque on the impeller is measured in Newton-meters (Nm) in real-time during the wet granulation process. A torque sensor is attached to the impeller shaft and therefore, a direct measurement of the force to turn the impeller is possible rather than indirect or calculated value (4). A direct coupled motor gives a accurate reading.
However, each of these methods have their own pros and cons and therefore, the selection of a suitable method is based on the application, the availability of the required facilities and the cost incurred. Fig.1 enlists the several techniques used for end point detection along with advantages and disadvantages of each of them.
Conclusion
To conclude, with the conventional pharmaceutical manufacturing unit operations, though there are several techniques evolved over time for endpoint detection, accuracy still poses as a challenge. The traditional techniques do not provide exact end-point determination and the newer techniques are relatively expensive. The area still has scope for development of a sensitive, cost-effective technique giving reproducible results.
From a technical and practical point of view, it is very difficult to arrive at a perfect end point with these methods to ensure batch-to-batch reproducibility. There are high chances of over wetting, if there the binder addition method and rate are not optimized. Due to multiple processes occurring simultaneously, a scientific endpoint is really blur. Therefore, agranulation end point is always based on the formulation, process, batch, and the desired granule properties. End-point detection is an everyday affair within a pharmaceutical industry during the product development and achieving the same is possible by either of the methods.

