The Gansons Pharma Integrated Granulation Line
Wet granulation remains the most widely used process for production of pharmaceutical solid-dosage forms.
Modern production processes demand an integrated, scalable, reproducible, and risk-free manufacturing solution.
The Gansons Integrated Granulation Suite, called the Force Multiplier® is the preferred wet-granulation solution for the world’s fastest growing regulated pharmaceutical companies.
The Gansons Force Multiplier® facilitates a high, predictable, reproducible throughput with minimized downtime and advanced safety features.
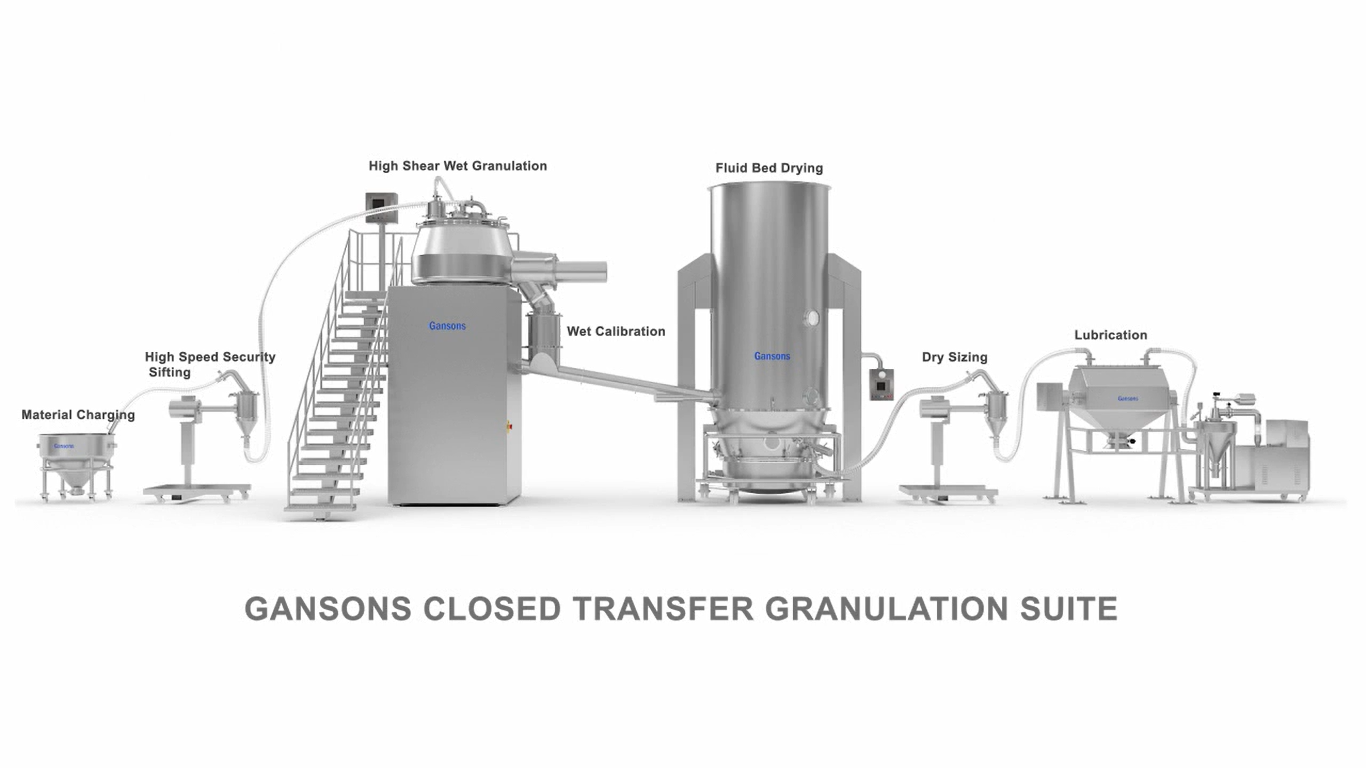
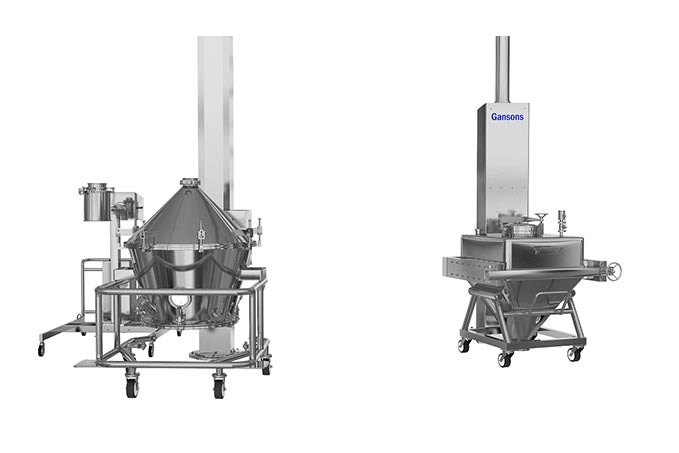
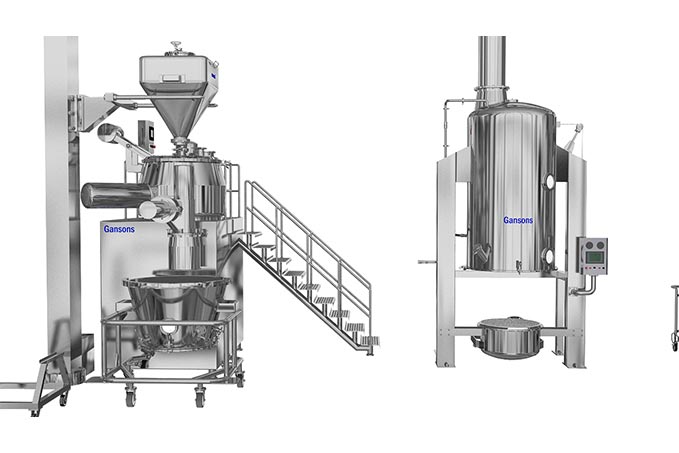
The Gansons Pharmaceutical Integrated granulation line including High speed Mixer & Granulator, Inline Wet mill, Fluid Bed Dryer & Granulator, Lifting device with Inline Dry Mill, and IBC Lift Blender at different sizes depending on customer demand.
Increased Output
• Compact footprint, line-balanced for maximum throughput
• Manufacturing continuum achieved
Intelligent Automation
• Centralized automation platform drives reproducible results
• Reduced human intervention means negligible mistakes and therefore, fewer delays
• 21-CFR Part 11 compliance for data integrity and storage, ALCOA+ compliant
• Pharma 4.0 enabled software, IoT enabled
• Smart graphs for lot reproducibility
• Responsive, ergonomic, intuitive interface
• 3-modes with multi-level password access
Improved Safety
• Closed transfer of materials ensures minimal operator exposure
• Safety interlocks
• 12-Bar shock-resistant designs available for structural integrity and containment.
• ATEX rated (flame proof) designs based on zoning requirements. All components are by default compliant to a Zone 2/22 production environment.
• All contact parts in SS 316L, or FDA approved materials (gaskets, seals, filter bags etc.)
Predictable scalability from R&D to production
Cleaning convenience
WIP system for in-situ cleaning (can be recipe driven)
Fast changeover
Site Installations
Compliances and Certifications